Atlanta, GA–Parents and other caregivers of infants should stop using bobbie Milk-Based Powder Companion Formula from Bobbie Baby Inc. This product was manufactured in, Germany and imported into the United States. The formula was sold online by Bobbie Baby Inc. through the firm website.
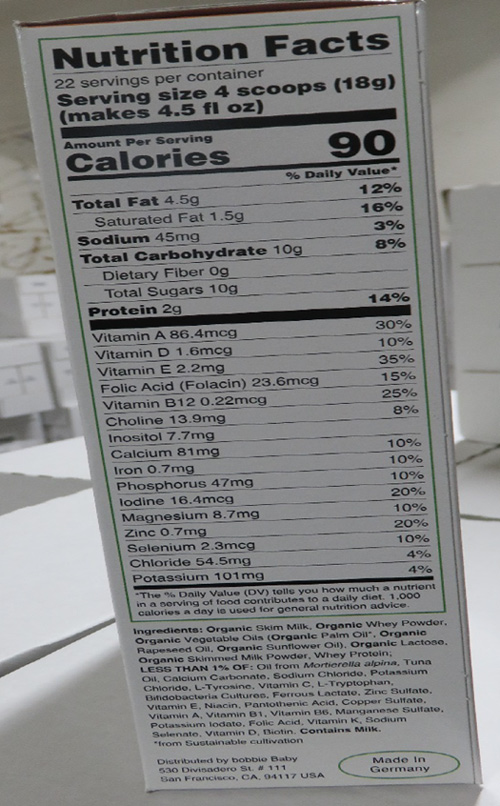
Based on the current information provided to the FDA by the firm, products were sold and distributed only to consumers in the San Francisco Bay Area of California. The FDA advises parents and caregivers to stop using and buying this formula because these products do not provide adequate nutrient levels for some infants, particularly for infants born prematurely or with a low birth weight, having low iron levels at birth or at risk for becoming iron deficient due to illness. Inadequate intake of iron during infancy may lead to iron deficiency anemia, which, if untreated, has irreversible cognitive and functional development outcomes.

This product also lacks the labeling for amounts of the following nutrients required in infant formula: linoleic acid, vitamin K, thiamine, riboflavin, vitamin B6, niacin, pantothenic acid, biotin, vitamin C, copper, iodine, and manganese. The absence of any of these key nutrients in infant formula may lead to poor growth, nutrient deficiencies, and/or serious health problems for developing infants.
Product
- Product Name: Bobbie Milk-Based Powder Companion Formula, Net wt. 14.1 oz (400g)
- Packaging: Brown and green cardboard boxes (2, 4, or 8 units per box) with the bobbie logo
- Lot numbers: Codes on the product of concern are; L6236501Z001; Use By: 9.15.2020; L6236501Z002; Use By: 9.15.2020; L6236501Z003; Use By: 9.16.2020; L6236501Z004; Use By: 9.16.2020
- Dates: Product of concern was shipped between the dates of May 12, 2019 and May 30, 2019
Recently, the FDA conducted an inspection of Bobbie Baby Inc., and determined that the firm is not in compliance with FDA regulations instituted to ensure the health of developing infants who consume infant formula. The firm was inspected under the Infant Formula Requirements (21 Part 106 and Part 107) included in Title 21 of the Code of Federal Regulations.
Review of the firm’s product labeling during the inspection revealed that the product listed above was being marketed as an infant formula.
These products have not been manufactured in compliance with infant formula regulations.(FDA)



















